When you pick up a prescription for a generic drug, you’re not getting a cheaper version of medicine-you’re getting the same medicine. But how does the FDA make sure that a $5 generic pill works just like its $50 brand-name cousin? It’s not magic. It’s science, strict rules, and thousands of tests done behind the scenes.
What Makes a Generic Drug Legally the Same?
The FDA doesn’t just approve generic drugs because they look alike or cost less. They have to prove they’re pharmaceutically equivalent and bioequivalent. That’s the two-part test every generic must pass.
Pharmaceutical equivalence means the generic has the exact same active ingredient, strength, dosage form (pill, injection, cream), and route of administration (oral, topical, etc.) as the brand-name drug. It also has to be labeled for the same uses. So if the brand-name drug treats high blood pressure, so does the generic. No surprises there.
But here’s where most people get confused: inactive ingredients can be different. That’s why a generic pill might be white instead of blue, or shaped differently, or have a different filler like cornstarch instead of lactose. Those don’t affect how the drug works. What matters is the active ingredient-how much of it gets into your bloodstream, and how fast.
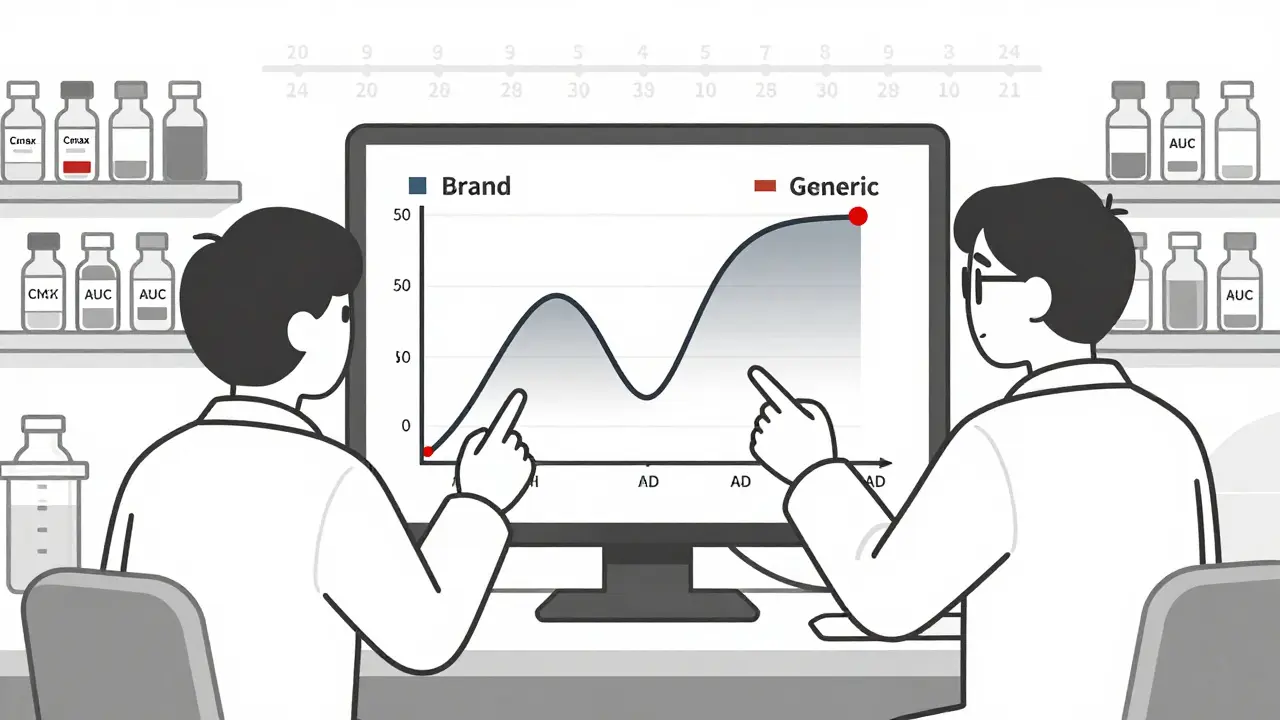
That’s where bioequivalence comes in. The FDA requires that the generic drug delivers the same amount of active ingredient into your blood at the same rate as the brand-name version. They measure this using two key numbers: Cmax (the highest concentration in your blood) and AUC (how much of the drug is absorbed over time). For approval, the 90% confidence interval for both must fall between 80% and 125% of the brand-name drug’s values. In plain terms? Your body absorbs the generic drug within 20% of how it absorbs the brand. That’s considered clinically the same.
How Are Generic Drugs Tested?
Testing doesn’t stop at the lab. The FDA demands real-world data from human studies. For most small-molecule generics-like antibiotics, blood pressure pills, or antidepressants-companies run single-dose studies with 24 to 36 healthy volunteers. Blood samples are taken over hours to map how the drug moves through the body. If the curves match up closely enough, the drug gets approved.
But not all drugs are that simple. Complex products like inhalers, injectables, or topical creams can’t be judged by blood levels alone. For these, the FDA has developed over 2,100 product-specific guidances. These outline extra tests: particle size analysis for inhalers, viscosity and spreadability for creams, or release rates for long-acting injections. These are called Q3 sameness tests-meaning the generic must match the brand in quality, performance, and characteristics, not just chemistry.
Stability testing is another must. Generics have to prove they won’t break down or lose potency over time. Manufacturers run accelerated tests-keeping samples at 40°C and 75% humidity for six months-and long-term studies at normal storage conditions for 12 to 24 months. If the drug still meets its specs after two years, it gets a two-year shelf life. No shortcuts.
Manufacturing Rules: No Exceptions
It doesn’t matter if you’re making a generic or a brand-name drug-the same rules apply. The FDA enforces Current Good Manufacturing Practices (cGMP), written in 21 CFR Parts 210 and 211. These cover everything: how raw materials are stored, how machines are cleaned, how batches are tested, and how records are kept.
Every batch of a generic drug must be tested for identity, strength, purity, and quality before it leaves the factory. The methods used must be validated-proven to give accurate, repeatable results. And the FDA doesn’t just trust the paperwork. They send inspectors to over 3,500 facilities every year-some in the U.S., many overseas in India, China, and elsewhere. A single failed inspection can delay approval for months.
Here’s something most people don’t know: about half of all generic drugs in the U.S. are made by the same companies that make the brand-name versions. Often, they’re made in the same factory, on the same lines. The only difference is the label.
The ANDA Process: How Long Does It Take?
Getting a generic approved isn’t fast. Companies submit an Abbreviated New Drug Application (ANDA), which skips the costly animal and human safety trials because they’re relying on the brand-name drug’s existing data. But the FDA still reviews every detail: chemistry, manufacturing, bioequivalence studies, labeling, and facility compliance.
Under the Generic Drug User Fee Amendments (GDUFA), the FDA aims to review a complete ANDA in 10 months. But many applications need revisions. In 2022, the FDA issued 478 complete response letters-essentially, “here’s what’s missing”-and approved 892 original ANDAs. That means most generics go through at least one round of back-and-forth before approval.
For complex drugs, the process is even longer. The FDA’s Pre-ANDA program lets companies meet with regulators early to avoid costly mistakes. In 2022 alone, over 1,200 of these meetings happened. That’s not bureaucracy-it’s prevention.
Why Do Some People Say Generics Don’t Work?
Most people don’t notice a difference. A 2022 Consumer Reports survey found 89% of users were satisfied with generics, and 62% chose them to save money. On Reddit’s pharmacy forum, 83% of 1,400+ respondents said they saw no difference between brand and generic.
But there are exceptions. Drugs with a narrow therapeutic index-where even a small change in blood level can cause harm-are trickier. Levothyroxine, used for thyroid conditions, is one. A 2021 JAMA study found that 12.3% of patients switching between different generic versions had thyroid hormone levels that shifted enough to require a dose adjustment. That’s why doctors sometimes stick with one brand or generic for these patients.
Same goes for seizure meds like phenytoin or blood thinners like warfarin. A few patients report changes in effectiveness after switching. The FDA acknowledges this and continues to refine testing methods for these high-risk drugs. But for the vast majority of medications-antibiotics, statins, antihistamines, pain relievers-switching generics is safe and effective.
The Bigger Picture: Cost and Access
Generic drugs save the U.S. healthcare system an estimated $37 billion every year. In 2022, generics made up over 90% of all prescriptions filled-but only about 23% of total drug spending. That’s the power of competition. Without generics, many people couldn’t afford their meds.
The market is growing fast. It was worth $135.7 billion in 2022 and is projected to hit $180.3 billion by 2027. The FDA’s Office of Generic Drugs has 750 staff members dedicated to reviewing these applications. That’s more than ever before.
And the agency isn’t stopping. In its 2023-2027 strategic plan, the FDA allocated $15.7 million to develop better testing tools for complex generics-like biosimilars and non-biological complex drugs. They’ve held 18 public workshops since 2017, bringing together scientists, manufacturers, and patient advocates to solve real problems.
Dr. Janet Woodcock, former head of the FDA’s drug center, put it simply: "FDA-approved generic drugs have the same high quality, strength, purity and stability as brand-name drugs." And she’s right. The system isn’t perfect, but it’s built on science, not speculation.
What You Should Know Before Taking a Generic
- If your doctor prescribes a brand-name drug, ask if a generic is available. It’s almost always safe.
- For drugs like thyroid meds, seizure control, or blood thinners, stick with the same generic brand unless your doctor advises otherwise.
- If you notice a change in how you feel after switching generics, talk to your pharmacist or doctor. It’s rare, but it happens.
- Don’t assume a more expensive generic is better. Price doesn’t reflect quality here.
- Check the FDA’s website for approved generics-there’s a public database you can search.
Generic drugs aren’t a compromise. They’re the result of one of the most carefully regulated systems in modern medicine. The FDA doesn’t cut corners to save money. It holds generics to the same standard it holds the originals. And that’s why you can trust them.
Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generic drugs to prove they are pharmaceutically and bioequivalent to the brand-name version. This means they deliver the same active ingredient at the same rate and extent into your bloodstream. Over 90% of prescriptions in the U.S. are filled with generics, and studies consistently show they work just as well for most conditions.
Why do generic drugs look different from brand-name drugs?
By law, generics can’t look exactly like the brand-name drug because of trademark rules. That’s why color, shape, size, or scoring may differ. But the active ingredient, strength, and dosage form are identical. The differences are only in inactive ingredients like dyes or fillers, which don’t affect how the drug works.
Can I switch between different generic brands?
For most drugs, yes. But for medications with a narrow therapeutic index-like levothyroxine, warfarin, or phenytoin-switching between different generic versions can sometimes cause small changes in blood levels. If you’re on one of these, talk to your doctor before switching. Consistency matters more than cost in these cases.
Are generic drugs made in the same facilities as brand-name drugs?
Yes. About half of all generic drugs in the U.S. are made by the same companies that produce brand-name versions, often in the same factories. The FDA inspects all facilities-brand and generic-using the same strict standards. The label is the only difference.
How does the FDA ensure quality in overseas generic drug factories?
The FDA inspects over 3,500 manufacturing sites worldwide each year, including facilities in India, China, and other countries. They use the same cGMP rules regardless of location. If a facility fails inspection, the FDA can block imports or delay approval. No foreign manufacturer gets a pass.
Do generic drugs expire faster than brand-name drugs?
No. Generic drugs must prove stability over the same shelf life as the brand-name version-usually 12 to 24 months. Manufacturers run accelerated and long-term stability tests to ensure the drug remains potent and safe until the expiration date. The expiration date is based on real data, not guesswork.
Are there any drugs that don’t have generics?
Yes. Some drugs are still under patent protection, making generics illegal until the patent expires. Others are too complex to replicate with current technology-like certain biologics or inhalers with intricate delivery systems. But for the vast majority of medications, generics are available.


1 Comments
Wow, i had no idea generics had to go through all that testing! i always thought they were just copied with cheaper stuff. the bioequivalence thing with Cmax and AUC is wild-like, they’re literally measuring how your body absorbs it. mind blown 🤯