
Every year, millions of Americans pick up prescriptions at the pharmacy, often without realizing their brand-name drug has been swapped for a cheaper generic version. In most states, they don’t need to give permission for it. This isn’t a secret trick or a glitch in the system-it’s presumed consent, a legal framework that lets pharmacists substitute generic drugs without asking, as long as the drugs are considered therapeutically equivalent. It’s designed to save money, speed up service, and keep prescriptions affordable. But it’s not the same everywhere. And for some patients, especially those on critical medications, it can carry real risks.
How Presumed Consent Works
Presumed consent means the law assumes you’re okay with getting a generic drug instead of the brand-name one your doctor wrote. You don’t have to say yes. You don’t even have to be asked. The pharmacist just does it-unless you’ve specifically told them not to, or your state says they can’t.
This system started gaining ground after the 1984 Hatch-Waxman Act made it easier for generic drugs to be approved. The FDA now rates every generic drug in its Orange Book with an ‘A’ rating if it’s bioequivalent to the brand-name version. That means it delivers the same amount of active ingredient into your bloodstream at the same rate. For most drugs, that’s enough to guarantee the same effect.
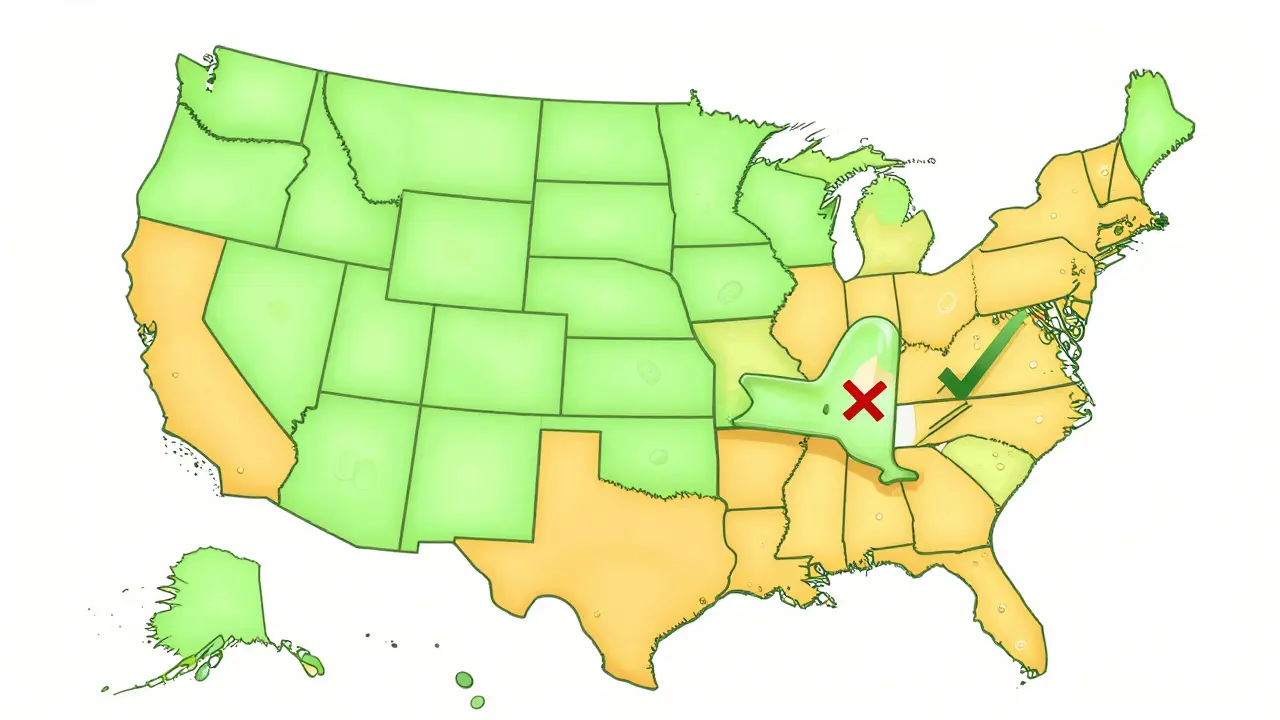
In 43 states and Washington, D.C., pharmacists are legally allowed to make the switch without asking. That’s the majority. But in seven states-Alaska, Delaware, Hawaii, Maine, Maryland, New Mexico, and West Virginia-you must give explicit consent before a substitution happens. Even in presumed consent states, pharmacists often have to notify you after the fact, either on the label, through a handout, or via a digital alert.
Why This System Exists
The goal is simple: cut costs without cutting care.
Generic drugs make up 90% of all prescriptions filled in the U.S. But they account for only about 15% of total drug spending. That’s because they’re cheaper-often 80-85% less than brand-name versions. Over the last decade, generic substitutions have saved the U.S. healthcare system roughly $1.68 trillion, according to the Association for Accessible Medicines. That’s billions in savings for patients, insurers, and Medicare.
For patients on long-term medications-like blood pressure pills, cholesterol drugs, or diabetes treatments-the difference can be hundreds of dollars a year. One patient in California told Drugs.com she saved $45 a month just by switching to a generic. For seniors on fixed incomes, that’s not a luxury-it’s a necessity.
Pharmacies benefit too. Presumed consent cuts down on time spent asking for permission. A 2022 study by the American Society of Health-System Pharmacists found that pharmacists save about 1.7 minutes per prescription. Multiply that across millions of fills, and you’re looking at $2.8 billion in annual labor savings.
Where the System Gets Complicated
Not all drugs are created equal. While most generics work just like their brand-name counterparts, some medications have what’s called a narrow therapeutic index-meaning the difference between a safe dose and a dangerous one is very small.
Drugs like warfarin (a blood thinner), levothyroxine (for thyroid issues), and certain antiepileptic medications fall into this category. Even tiny variations in how the body absorbs the drug can lead to serious problems: a stroke, a seizure, or a thyroid crisis.
The American Epilepsy Society documented 178 cases of breakthrough seizures between 2018 and 2022 linked to generic substitutions. In response, 15 states-including Tennessee, Hawaii, and New York-now require explicit consent before substituting these drugs. Some states even ban substitution entirely for certain medications.
And it’s not just epilepsy drugs. Pharmacists in presumed consent states must still check state-specific restrictions before swapping. One pharmacist in Ohio reported that while 95% of patients never notice the switch, the other 5% often become suspicious. “They think we’re cutting corners,” he said on Reddit. “It’s hard to rebuild trust once it’s broken.”

Biosimilars: The New Frontier
Generics are small-molecule drugs-simple chemical compounds. But newer drugs called biologics are much more complex. They’re made from living cells, not synthesized in a lab. Examples include insulin, rheumatoid arthritis treatments, and cancer drugs.
Similar versions of biologics are called biosimilars. They’re not exact copies, but they’re close enough to be approved as interchangeable. The problem? Presumed consent doesn’t always apply.
As of 2023, only six states-Arizona, Indiana, Iowa, Massachusetts, New Jersey, and Pennsylvania-allow automatic substitution of interchangeable biosimilars without consent. Four states-North Carolina, Oklahoma, Pennsylvania, and Texas-ban it entirely. Another 35 states have unclear or restrictive rules.
That creates a mess for patients and pharmacists. A patient in New York might get a biosimilar automatically. The same patient, if they move to Texas, could be denied the switch-even if their doctor and pharmacist agree it’s safe.
The FDA’s Purple Book lists which biosimilars are interchangeable, but pharmacists must check state laws before acting. Many still don’t have the training or tools to keep up.
What Patients Need to Know
If you’re on a chronic medication, don’t assume your prescription will always be filled the same way. Here’s what you should do:
- Ask your pharmacist whether your medication was switched, even if you didn’t get asked.
- Check the label. Generic drugs have different names, but the active ingredient should match. If you’re unsure, ask.
- Know your drug class. If you take antiseizure meds, blood thinners, thyroid medicine, or certain psychiatric drugs, be extra cautious. Ask if substitution is allowed in your state.
- Speak up. You have the right to refuse a generic. Say: “I want the brand name as prescribed.”
- Keep a log. Write down the name of each drug you get, brand or generic. If you notice a change in how you feel, it helps your doctor track it.
Most patients never have an issue. But for those who do, the consequences can be life-changing. One patient in Tennessee wrote on Drugs.com: “My seizure medication stopped working after substitution.” He spent weeks in the hospital. His story isn’t rare.
What Pharmacists Are Doing About It
Pharmacists aren’t trying to trick anyone. They’re caught between cost-saving laws and patient safety.
In presumed consent states, pharmacists spend an average of 42 seconds per prescription documenting substitutions-longer than in states requiring consent. They use tools like the American Pharmacists Association’s online substitution calculator and state pharmacy board guidelines to stay compliant.
Independent pharmacies struggle more than big chains. Chain pharmacies have automated systems that flag restricted drugs and auto-populate consent forms. Independent pharmacists often rely on memory, printed manuals, or calls to state associations.
According to a 2023 survey by the National Community Pharmacists Association, 78% of pharmacists in presumed consent states feel confident in their ability to follow the rules. But 41% say special restrictions for narrow therapeutic index drugs are confusing and inconsistently enforced.
The Future of Presumed Consent
There’s growing pressure to fix the system’s flaws.
New York passed a rule in March 2023 requiring electronic documentation of all substitutions. California expanded notification rules for biosimilars in 2022. The Uniform Law Commission is pushing a Model State Substitution Act that would create a standardized framework across states-especially for high-risk drugs.
The proposed solution? A tiered consent model. Keep presumed consent for most drugs-blood pressure pills, statins, antibiotics-but require explicit consent for narrow therapeutic index drugs and biosimilars. That way, you get the cost savings without the risk.
Industry analysts predict that by 2028, biosimilars will make up 25% of the biologics market. That means every state will need to update its laws. The question isn’t whether presumed consent will survive-it’s whether it will evolve to protect patients better.
Bottom Line
Presumed consent saves money. It saves time. It works for most people, most of the time. But it’s not foolproof. If you’re on a critical medication, don’t assume everything’s fine. Ask questions. Know your rights. And if something feels off after a switch-tell your doctor. Your health isn’t a cost-saving metric. It’s personal. And you deserve to be in control.



9 Comments
So let me get this straight-we’re okay with letting pharmacists swap life-saving meds without asking, but if you try to switch your coffee brand without asking, people freak out? 🤦♀️ This isn’t convenience, it’s medical negligence dressed up as ‘cost-saving.’ If I were on warfarin and got a generic that made me clot, would you still call it ‘smart economics’? No. You’d call it a crime. And yet here we are, letting pharmacies gamble with lives because someone’s insurance premium went up. Wake up, America.
Oh, how *delightfully* capitalist. Let’s just assume consent because money talks and patients whisper. The FDA’s Orange Book is basically a bingo card for pharmaceutical fraud-‘A’ rated? Sure, if you ignore the 0.02% variance in bioavailability that triggers seizures in 1 in 10,000 patients. And let’s not forget the 41% of pharmacists who admit they’re confused by state rules. That’s not a system. That’s a liability lottery. Someone’s going to die. And when they do, we’ll all shrug and say, ‘Well, it saved $45 a month.’
It’s funny how the same people who scream about government overreach are fine with pharmacists making medical decisions without consent. This isn’t socialism-it’s smart business. If you can’t afford your meds, you’re not ‘entitled,’ you’re just bad at budgeting. The system works for 95% of people. The other 5%? They should’ve read the fine print. America doesn’t owe you brand-name drugs because your doctor felt fancy. Take the generic, be grateful, and stop whining.
It’s wild how we treat our bodies like disposable consumer goods. We’ll spend $200 on a yoga mat that ‘aligns our chakras’ but won’t pay $15 extra to make sure our seizure meds don’t suddenly stop working. 🤔 We’ve outsourced our health to efficiency algorithms and wonder why we feel so disconnected. Maybe the real problem isn’t the law-it’s that we’ve stopped seeing ourselves as people who deserve to be asked. Just saying… 🌱
Most people don’t realize that generics are required by law to be within 80–125% bioequivalence range-that’s a 45% swing in absorption. For levothyroxine, that’s like going from 100mcg to 145mcg. That’s not ‘equivalent,’ that’s a different drug. The FDA’s Orange Book is a joke. I’ve seen patients go from euthyroid to myxedema coma after a switch. And no, pharmacists don’t always know this. They’re not doctors. They’re cashiers with white coats.
I’ve been on the same generic for 8 years. Never had an issue. My blood pressure is stable. My cholesterol is down. I don’t care if it’s brand or generic as long as it works. People who freak out about this are either paranoid or got scammed by a pharma ad. Stop turning medicine into a cult. If it’s FDA-approved, take it. Move on.
Hey, just a heads up-if you're on levothyroxine or warfarin, always check the label. The generic name might be different (like levothyroxine sodium vs Synthroid), but the active ingredient is the same. I’m a pharmacist and I always tell patients to write down the pill color and imprint code. If it changes and you feel weird? Call your doc. Don’t wait. Also, you can always say ‘no substitution’ on the script. It’s your right. 💙
There’s a deeper question here: when did we decide that medical care should be optimized for efficiency instead of dignity? We don’t let banks swap your account number without asking. We don’t let mechanics swap your engine without telling you. But we let a stranger at a counter swap your brain chemistry? We’ve normalized vulnerability. And now we call it progress. Maybe the real crisis isn’t the law-it’s that we’ve forgotten how to say ‘no’ without feeling guilty.
I just want to say that this whole system isn’t perfect, but it’s not the monster some people make it out to be. For every story of someone having a bad reaction, there are ten thousand people who got their insulin for $10 instead of $300 and didn’t have to choose between food and medicine. That’s real. That’s human. Yes, we need better rules for narrow therapeutic index drugs-absolutely. But don’t throw out the baby with the bathwater. We can fix the gaps without scrapping the whole thing. Let’s push for tiered consent, better training, and clearer labeling. Let’s make it smarter, not scarier. We can do this, together. 🤝