SGLT2 Inhibitor Fracture Risk Calculator
Personal Health Profile
Answer these questions to get personalized fracture risk estimates.
Estimated Fracture Risk
Your fracture risk score:
Based on your health profile...
Canagliflozin (300 mg)
Fracture risk: N/A per 1,000 person-years
Hazard ratio: N/A
Warning: This drug is associated with increased fracture risk for people with risk factors.
Canagliflozin (100 mg)
Fracture risk: N/A per 1,000 person-years
Hazard ratio: N/A
Empagliflozin (10/25 mg)
Fracture risk: N/A per 1,000 person-years
Hazard ratio: N/A
Note: This drug shows no increased fracture risk.
Dapagliflozin (5/10 mg)
Fracture risk: N/A per 1,000 person-years
Hazard ratio: N/A
Note: This drug shows no increased fracture risk.
Important Note: This calculator is for informational purposes only. Always consult with your healthcare provider before making changes to your medication.
When you're managing type 2 diabetes, choosing the right medication isn't just about lowering blood sugar. It’s about balancing benefits with hidden risks - and one of the most misunderstood concerns is bone health. For years, doctors and patients have worried that SGLT2 inhibitors, a popular class of diabetes drugs, might increase the chance of broken bones. But the truth isn’t as simple as it sounds. Some of these drugs carry a real risk. Others don’t. And knowing the difference could change how you or a loved one takes your medication.
What Are SGLT2 Inhibitors?
SGLT2 inhibitors are a group of oral diabetes medications that work in the kidneys. Instead of forcing the body to make more insulin or making cells more sensitive to it, they tell the kidneys to dump extra sugar into the urine. That’s it. This simple trick lowers blood glucose without causing weight gain or low blood sugar - two common side effects of older diabetes drugs.
The first one, dapagliflozin (Farxiga), got FDA approval in 2013. Soon after, canagliflozin (Invokana) and empagliflozin (Jardiance) followed. Today, these drugs are prescribed to millions worldwide. But they’re not just for blood sugar. Large studies like EMPA-REG OUTCOME and DECLARE-TIMI 58 showed they also protect the heart and kidneys. That’s why many endocrinologists now consider them first-line for patients with heart failure, chronic kidney disease, or a history of heart attacks.
The Fracture Risk That Started It All
The trouble began in 2015. Data from the CANVAS trial - a major study testing canagliflozin - showed a 26% higher risk of fractures compared to placebo. That wasn’t a small blip. It was statistically significant. The FDA took notice. By May 2016, they added a formal warning to canagliflozin’s label: “Fractures occurred as early as 12 weeks after starting treatment.” Most were from minor falls, like tripping off a curb or slipping in the shower.
What made this worse? The fractures weren’t random. They happened mostly in the arms, hands, and feet - areas that don’t usually break unless there’s a direct impact. And they happened more often in women and older adults. The numbers weren’t huge - about 15 fractures per 1,000 people per year on canagliflozin versus 12 on placebo - but for someone already at risk for osteoporosis, even a small increase matters.
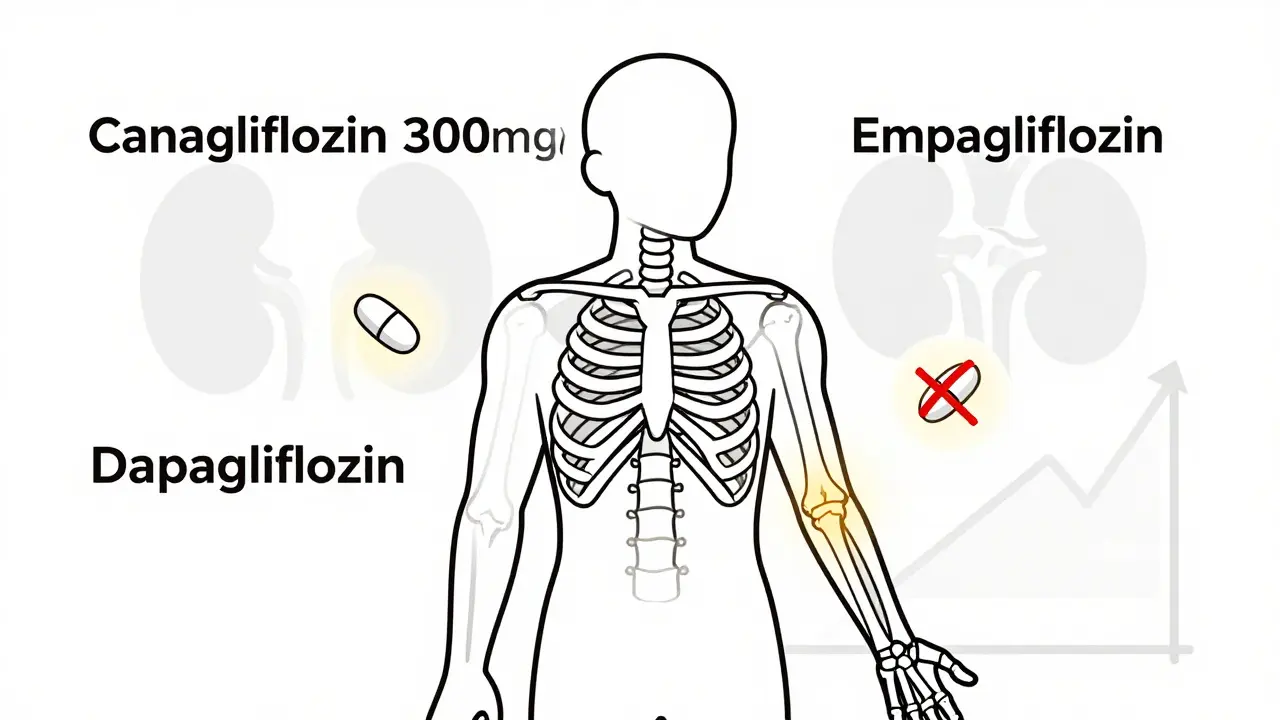
Not All SGLT2 Inhibitors Are the Same
This is where things get critical. The fracture risk isn’t a class-wide problem. It’s specific to canagliflozin - and even then, mostly at the 300 mg dose.
Empagliflozin (Jardiance) and dapagliflozin (Farxiga) didn’t show the same signal in their own major trials. EMPA-REG OUTCOME tracked over 7,000 people for more than three years. DECLARE-TIMI 58 followed nearly 17,000 for five years. Neither found a meaningful increase in fractures. A 2023 meta-analysis of 27 clinical trials involving over 20,000 patients found no overall link between SGLT2 inhibitors and fractures - but when researchers pulled out canagliflozin, the risk popped back up.
Here’s what the data says:
| Drug | Dose | Fracture Rate | Hazard Ratio (vs Placebo) |
|---|---|---|---|
| Canagliflozin | 300 mg | 15.4 | 1.26 |
| Canagliflozin | 100 mg | 12.8 | 1.08 |
| Empagliflozin | 10 mg / 25 mg | 11.5 | 0.98 |
| Dapagliflozin | 5 mg / 10 mg | 11.2 | 0.99 |
That’s not a typo. Empagliflozin and dapagliflozin actually had slightly lower fracture rates than placebo. Canagliflozin is the outlier.
Why Does Canagliflozin Affect Bones?
It’s not one thing - it’s a mix. Researchers have found several possible reasons:
- Weight loss: SGLT2 inhibitors cause modest weight loss - around 2-4 kg on average. That sounds good, but losing weight can also mean losing bone mass. However, studies show weight loss only explains about 3% of the bone density drop.
- Phosphate shifts: These drugs cause more phosphate to be reabsorbed by the kidneys. That tricks the body into releasing parathyroid hormone and FGF23, both of which can pull calcium out of bones.
- Hormone changes: In women, canagliflozin 300 mg lowered estradiol levels by 9.2%. Estradiol protects bone density. A drop like that could matter, especially in postmenopausal women.
- Low blood pressure: SGLT2 inhibitors can cause dizziness or fainting due to low blood pressure when standing up. That increases fall risk - and falls cause fractures.
- Bone density loss: In a two-year FDA-mandated trial, patients on canagliflozin lost 0.92% of bone density at the hip and 1.04% at the spine. Placebo users lost less than half that. That’s a measurable difference.
Other SGLT2 inhibitors don’t show these same effects to the same degree. That’s why the FDA only warns about canagliflozin.
Who Should Avoid Canagliflozin?
If you’re at high risk for fractures, canagliflozin isn’t the best choice. That includes:
- People with osteoporosis (T-score ≤ -2.5)
- Those with a prior fracture, especially after age 50
- Women over 65 with low estrogen
- Anyone with poor balance, vision problems, or who takes sedatives
- People on long-term steroids or with rheumatoid arthritis
The American Association of Clinical Endocrinologists and the American Geriatrics Society’s Beers Criteria both recommend avoiding canagliflozin in these groups. Other SGLT2 inhibitors like empagliflozin and dapagliflozin are still considered safe.
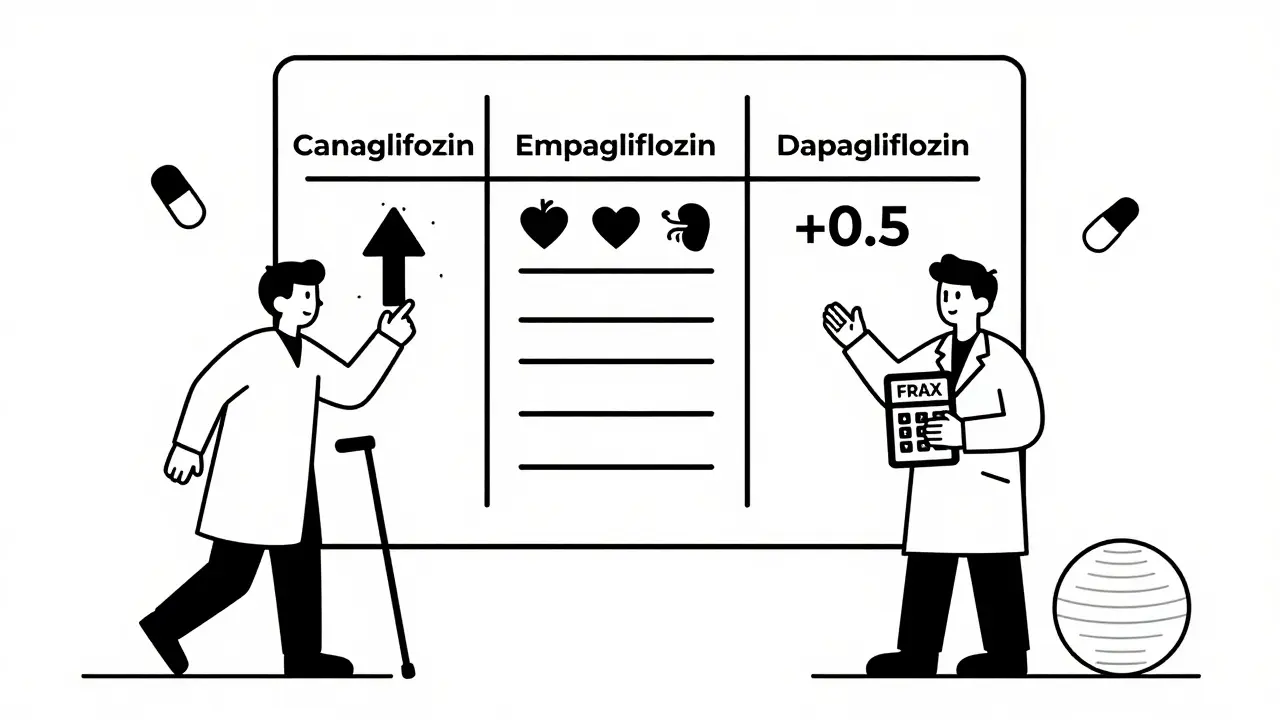
Doctors now use tools like FRAX (Fracture Risk Assessment Tool) to estimate risk. The 2023 American Diabetes Association guidelines add 0.5 points to your FRAX score - but only if you’re taking canagliflozin. That small bump can push someone from “low” to “moderate” risk, which changes treatment decisions.
What About Bone Density Tests?
Should you get a DXA scan before starting an SGLT2 inhibitor?
For canagliflozin - yes, if you have any risk factors. The American College of Endocrinology recommends a bone density scan if you’re over 65, have had a fracture, have low body weight, or are on long-term steroids. If your T-score is below -2.0, they advise choosing a different drug.
For empagliflozin or dapagliflozin? Routine scans aren’t needed unless you already have osteoporosis or other risk factors. The risk isn’t there.
And don’t forget: diabetes itself harms bone health. High blood sugar weakens bone structure over time. So even if you’re on a safe SGLT2 inhibitor, your overall fracture risk might still be higher than someone without diabetes.
What Do Real Doctors Think?
A 2022 survey of 347 endocrinologists showed that 68% adjust their prescriptions based on fracture risk. But here’s the split:
- 82% avoid canagliflozin in patients with osteoporosis
- Only 34% avoid dapagliflozin for the same reason
Some doctors still worry. Dr. Robert Heaney, a leading bone expert, says the number of fractures in trials was too low to be sure. He believes longer studies are needed.
But others, like Dr. Mary Buettner and Dr. Thomas Addison, reviewed real-world data from tens of thousands of patients and found no connection between SGLT2 inhibitors and fractures - except for canagliflozin. Their conclusion: “Concerns have been largely overstated.”
And here’s something surprising: a 2023 study in JAMA Network Open found that SGLT2 inhibitors had lower fracture rates than GLP-1 receptor agonists (like semaglutide) and DPP-4 inhibitors (like sitagliptin) in high-risk patients. That’s right - some older diabetes drugs might be riskier than the newer ones.
What Should You Do?
If you’re currently taking canagliflozin and you’re healthy, with no history of fractures or osteoporosis - you’re probably fine. Don’t stop your medication without talking to your doctor.
If you’re starting a new diabetes drug and you’re over 65, have had a fracture, or have low bone density - ask your doctor:
- Is canagliflozin the best choice for me?
- Have I had a bone density scan?
- Would empagliflozin or dapagliflozin be safer for my bones?
- Should I take calcium and vitamin D?
- Do I need physical therapy or balance training?
Don’t let fear stop you from using a drug that protects your heart and kidneys. But do make sure you’re on the right one for your body.
The Bottom Line
SGLT2 inhibitors are powerful tools for diabetes. But they’re not all the same. Canagliflozin carries a small but real fracture risk - especially in older adults and those with weak bones. Empagliflozin and dapagliflozin do not. The evidence is clear: canagliflozin is the only one you need to worry about.
For most people, the benefits of these drugs - heart protection, kidney protection, weight loss - far outweigh the risks. But if you’re at high risk for fractures, talk to your doctor. There’s a better option out there.


14 Comments
The distinction between canagliflozin and other SGLT2 inhibitors is crucial, and this post lays it out with remarkable clarity. As someone who works in global health policy, I appreciate how the data is contextualized without alarmism. Many developing countries are adopting these drugs for their cardiovascular benefits, and knowing which ones carry bone risks could prevent unintended harm in elderly diabetic populations.
It’s also worth noting that access to DXA scans remains limited in many regions - so clinical judgment based on risk factors becomes even more vital than biomarkers.
Well-researched and deeply responsible writing.
Thank you for this.
So let me get this straight - the only SGLT2 inhibitor that breaks bones is the one that’s cheapest? Coincidence? I work in pharma procurement in India, and yeah, canagliflozin dominates the generic market. Doctors prescribe it because it’s affordable. Patients take it because they can’t afford the alternatives.
Now we’re telling them it might crack their hips? That’s not medical advice - that’s a socioeconomic trap.
My grandma fell last year broke her wrist on the shower mat and they put her on canagliflozin because her sugar was up and she had heart issues
she’s fine now but i still wonder if it was the drug or just old age
I’ve been on Jardiance for three years now and my bone density is actually stable - I even started walking daily after my doc said I was low risk. This post made me feel way less anxious. I didn’t realize empagliflozin was the safe one - my doc just said ‘this one’s good for your heart.’
Thanks for breaking it down like this. I’m sharing it with my mom who’s on Invokana.
Also - why does everyone keep saying ‘fracture risk’ like it’s some sci-fi villain? It’s a number. A real one. With context. Stop panicking.
Oh great. Another American medical study that somehow makes everything more complicated than it needs to be. We have a drug that helps your heart, kidneys, and weight - and you’re telling me to avoid it because some women in their 70s might trip in the shower?
Meanwhile, in the UK, we just give people metformin and tell them to stop eating crisps. No scans. No hazard ratios. Just common sense.
Also - why is every study funded by Big Pharma? I bet if we looked at the funding sources, we’d find canagliflozin was the only one without a billionaire’s name on the grant.
Let’s be brutally honest - this entire narrative is a distraction. The real problem isn’t canagliflozin. It’s that doctors don’t read the full clinical trial data. They see ‘SGLT2 inhibitor’ and think ‘magic bullet.’
The FDA warning on canagliflozin is precise. It says ‘fractures occurred as early as 12 weeks.’ That’s not a vague association - it’s a temporal correlation with statistical power. Yet you still see it prescribed to 80-year-old women with osteoporosis.
And don’t get me started on the ‘other SGLT2 inhibitors are safe’ myth. The trials weren’t powered for fracture endpoints. You can’t prove a negative. This is basic epidemiology 101.
Anyone who says ‘empagliflozin is fine’ is either lazy or has a financial stake in the drug. The data is incomplete. The risk is unquantified. And you’re gambling with people’s bones.
Stop pretending this is settled science. It’s not. It’s a statistical gray zone with corporate marketing wrapped around it.
There is a deeper philosophical question here: when does a statistical signal become a moral imperative?
The 26% increased fracture risk with canagliflozin is small in absolute terms - 3 extra fractures per 1,000 person-years. But for an individual patient, that 3% is 100%.
Medicine is not just about population averages. It is about the person sitting across from the doctor, holding their coffee cup with a trembling hand, wondering if their next step might be their last.
Empagliflozin and dapagliflozin offer comparable cardiovascular protection without the same skeletal cost. To prescribe canagliflozin to a frail elderly woman is not negligence - it is a failure of moral imagination.
We must not confuse safety with absence of evidence. We must choose the path that honors the vulnerability of the patient - not the convenience of the formulary.
Oh, so now it’s just canagliflozin? Funny how the same company that made Invokana also owns the patents for the ‘safe’ ones. And the FDA only issued the warning after the lawsuit started. Coincidence? I think not.
Here’s what’s really happening: they knew canagliflozin caused bone loss but pushed it anyway because it was the first to market. Then they made the others with lower doses and cleaner trials. Same company. Same lab. Same profit motive.
They didn’t fix the problem - they just rebranded it.
And now you’re all acting like this is some scientific revelation? Wake up. It’s corporate triage. The elderly are the cost center. The heart and kidneys? That’s the marketing pitch.
Don’t trust the label. Don’t trust the trial. Trust nothing that has a patent number.
Oh darling, this is just the latest in a long line of pharmaceutical fairy tales. You know how many times we’ve been told a drug is ‘safe’ until someone’s hip shatters? I remember when they said Vioxx was fine. Then the hearts stopped. Then the lawsuits started.
And now we’re being told to trust a 2023 meta-analysis? Sweetheart, that was funded by the same consortium that gave us the ‘low-risk’ claims for estrogen patches in the 90s.
Meanwhile, my cousin’s mother broke her femur on canagliflozin - and her doctor said, ‘It’s just aging.’
But here’s the real tragedy: the patients who need these drugs most - the ones with heart failure and kidney disease - are the ones least able to afford a DXA scan. So they get the cheap one. And they break. And then we call it ‘natural progression.’
How very civilized of us.
So let me get this straight - you’re telling me I should avoid canagliflozin because of a 26% relative risk increase that only applies to people who already have a 1 in 1000 chance of breaking a bone per year?
Meanwhile, I’m on metformin and my blood sugar is still 180. I’m 55, I lift weights, I eat kale, and I’ve never broken a bone in my life. But now I’m supposed to switch to a drug that costs $400 a month just because some trial showed a tiny uptick in fractures among frail elderly women?
And you call this science? This is fearmongering dressed up as medicine. I’d rather take my chances with the cheap one and keep my heart healthy. My bones will be fine.
Okay but like… why is this even a thing? I just want my sugar down and my energy up. Why do I have to be a bone detective now? 🤡
Also I got my DXA scan last year and my T-score is -1.8 so I guess I’m just gonna keep taking canagliflozin because my doctor said ‘you’re fine’ and I trust her and also I don’t wanna pay for a new script 😭
As someone who grew up in rural Nigeria, I’ve seen how diabetes care shifts when access is limited. In urban centers, doctors have the luxury of choosing between drugs. In villages? They get what’s available - often the cheapest, most widely distributed option.
Canagliflozin is the most common SGLT2 inhibitor distributed in sub-Saharan Africa. The fracture risk is real - but so is the risk of uncontrolled diabetes: amputations, blindness, dialysis.
What’s the ethical choice here? To withhold a life-saving drug because a small subset might fracture? Or to provide it with better patient education and fall prevention?
This isn’t just about pharmacology. It’s about global equity. We need to fix the system - not just the prescription.
canagliflozin is bad for bones? lol no way that’s just the placebo effect or something. my uncle took it for 2 years and he broke his ankle falling off a ladder - but he was drunk and the ladder was broken. so like… it’s not the drug. it’s him.
also why are we even talking about this? i thought the point was to lower sugar not to turn everyone into a geriatric bone specialist. just take your pills and stop overthinking it.
Thank you for raising the global equity point - that’s exactly the conversation we’re not having. In Nigeria, we don’t have DXA machines in every clinic. But we do have community health workers. What if we trained them to assess fall risk? Simple tools: ask about past falls, check for vision impairment, test gait speed.
That’s not glamorous. It doesn’t make headlines. But it’s cheaper than a scan, and it saves lives.
And for patients on canagliflozin? A $2 pair of non-slip slippers and a nightlight could do more than any bone density report.
Medicine isn’t just about drugs. It’s about the whole person - and the whole world they live in.