When you take a pill, injection, or inhaler, you expect it to work exactly as it should-no matter when or where you use it. That’s not luck. It’s the result of strict stability testing, a process that makes sure medicines don’t break down before they reach you. At the heart of this process are two non-negotiable factors: temperature and time. Get these wrong, and a drug could lose potency, form harmful byproducts, or fail to dissolve properly. Regulatory agencies around the world enforce these conditions with precision because lives depend on it.
Why Temperature and Time Matter in Stability Testing
Stability testing isn’t just about watching a bottle sit on a shelf. It’s a controlled simulation of real-world conditions over years. The goal? To find out how long a medicine stays safe and effective. If a tablet degrades at 40°C, but your local pharmacy doesn’t have air conditioning, that’s a problem. If a liquid freezes during shipping and separates, that’s a problem. Stability testing catches these issues before the product hits the market. The science behind it is simple: heat and moisture speed up chemical reactions. In a drug, that means active ingredients can break down, excipients can absorb water, or containers can leach chemicals. Time is the variable that turns those small changes into big risks. That’s why regulators don’t just ask for “some data.” They demand exact conditions, over exact periods, with exact measurements.ICH Q1A(R2): The Global Standard for Temperature and Time
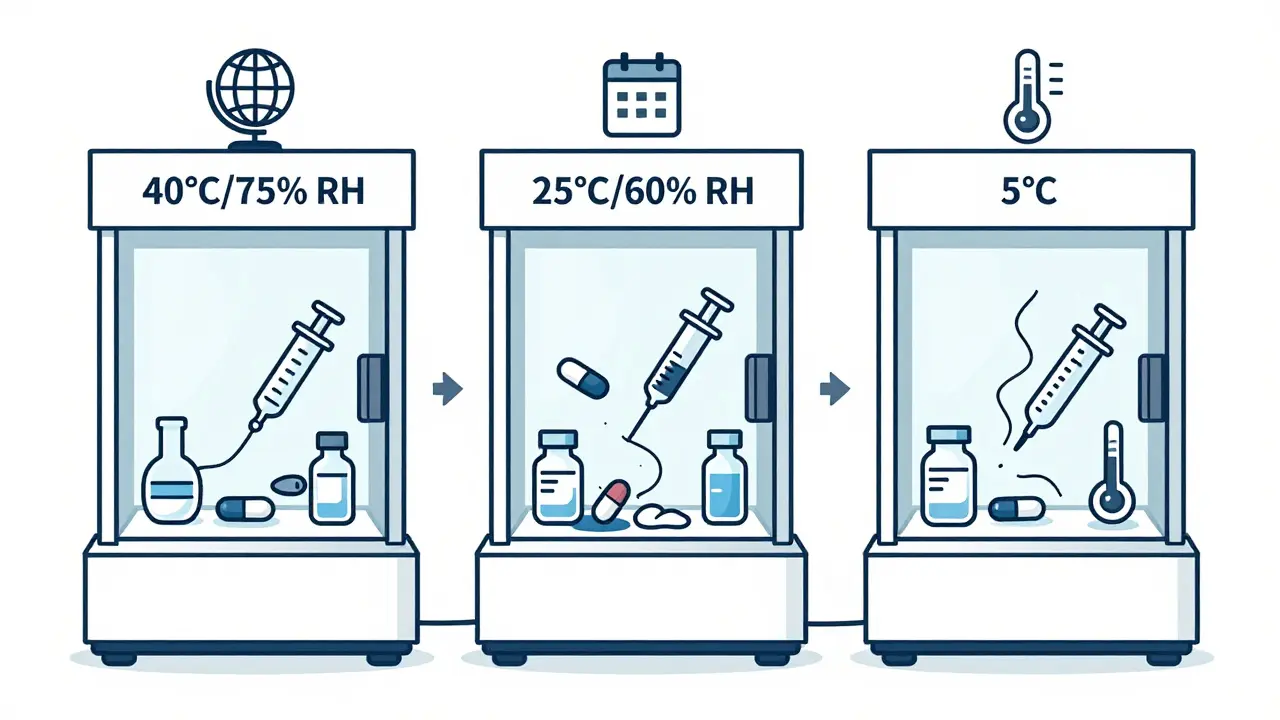
The International Council for Harmonisation (ICH) set the global benchmark in 2003 with ICH Q1A(R2). This isn’t a suggestion-it’s the rule in the U.S., Europe, Japan, Canada, Australia, and most major markets. It defines three key testing conditions, each with precise temperature and humidity targets.- Accelerated testing: 40°C ± 2°C and 75% RH ± 5% RH for 6 months. This isn’t meant to be realistic-it’s a stress test. If a drug fails here, it’s unlikely to survive real-world storage. This condition was chosen because it’s hot enough to accelerate degradation but not so hot that it melts common excipients.
- Long-term testing: Two options. Either 25°C ± 2°C and 60% RH ± 5% RH, or 30°C ± 2°C and 65% RH ± 5% RH. The choice depends on where the product will be sold. For example, a drug meant for tropical markets must be tested under the 30°C/65% RH condition.
- Intermediate testing: 30°C ± 2°C and 65% RH ± 5% RH for 6 months. Only required if long-term testing is done at 25°C and accelerated testing shows a “significant change.” This acts as a bridge between the two.
What About Refrigerated or Frozen Products?
Not all drugs are stored at room temperature. Insulin, many vaccines, and biologics like monoclonal antibodies need cold chains. For these, the rules change.- Refrigerated long-term: 5°C ± 3°C for 12 months. This simulates a home or clinic fridge.
- Refrigerated accelerated: 25°C ± 2°C and 60% RH ± 5% RH for 6 months. Notice: it’s not 40°C. That’s because freezing and thawing cycles are the real threat-not heat. Testing at 25°C mimics what happens if a vaccine is accidentally left out of the fridge for a few days.
How Long Do You Actually Have to Test?
The minimum data required at the time of regulatory submission varies by region. The FDA demands 12 months of long-term data. The EMA allows either 6 or 12 months, depending on which submission pathway you choose. This difference causes delays. A company submitting to both agencies might have to run two parallel studies-or wait longer for full data. Testing doesn’t stop at 12 months. Typical sampling points are at 0, 3, 6, 9, 12, 18, 24, and 36 months. Early time points (like 3 and 6 months) are critical because that’s when most degradation happens. If a drug shows a 5% drop in potency at 6 months, you can predict it’ll be below 90% by 24 months. That’s why statistical models like regression analysis are part of every stability protocol.Real-World Problems in Stability Testing
Even with perfect guidelines, things go wrong. A 2023 survey in the LinkedIn group for Pharmaceutical Stability Professionals found that 78% of labs had experienced a temperature excursion-meaning the chamber went outside the ±2°C tolerance. One incident can invalidate an entire 12-month study. That’s not just expensive-it delays patient access. Another issue: defining “significant change.” ICH Q1A(R2) says it’s when there’s a 5% change in assay, 10% in impurities, or failure to meet physical appearance or dissolution specs. But what counts as “failure”? One Pfizer quality analyst posted on Reddit about a 4.8% assay drop being rejected by regulators, even though the product was still within pharmacological tolerance. That’s the problem-subjectivity. Two labs can interpret the same data differently. Then there’s humidity. A 2022 AAPS paper found that 62% of stability failures in tablets came from humidity cycling-not constant high humidity. If a warehouse opens and closes doors daily, or a truck travels from dry Arizona to humid Florida, the moisture swings can cause tablet cracking, caking, or chemical breakdown. Standard tests use constant 60% RH. Real life doesn’t.What’s Changing in Stability Testing?
The ICH Q1A(R2) guidelines are nearly 20 years old. They were designed for traditional tablets and capsules. Today’s drugs are more complex: mRNA vaccines, antibody-drug conjugates, lipid nanoparticles. These break down in ways the old protocols don’t predict. The FDA is running a pilot program using Process Analytical Technology (PAT) to monitor drug stability in real time during manufacturing. If successful, this could cut testing time by 30-50% for continuous manufacturing products. Companies like Merck have already used intermediate testing to catch a polymorphic shift in Keytruda®-a change in crystal structure that could have affected how the drug was absorbed. Predictive modeling is also growing. Top pharmaceutical companies are running accelerated studies at 50-80°C to predict degradation in months instead of years. But regulators are cautious. The EMA rejected 8 model-based submissions in 2022-2023 because they couldn’t prove the models matched real-world behavior.
What Happens If You Don’t Follow the Rules?
Non-compliance isn’t a slap on the wrist. In 2022, the FDA issued 27 warning letters citing stability testing failures. One led to a recall of 150,000 vials of Teva’s Copaxone® after aggregation was found at 40°C. Another resulted in a complete halt of a new biologic’s approval because the company didn’t test under tropical conditions. The cost of failure? Millions in recalls, lost sales, and damaged reputation. The cost of doing it right? Around $185,000-$275,000 per product for a full stability program. But that’s still far cheaper than a recall.How to Get Started
If you’re developing a new drug, here’s what you need:- Choose your target markets. This determines your long-term storage condition (25°C/60% RH or 30°C/65% RH).
- Design your accelerated test: 40°C/75% RH for 6 months.
- Set up environmental chambers that maintain ±0.5°C and ±2% RH. Use temperature mapping to check for hot or cold spots.
- Define your sampling schedule: 0, 3, 6, 9, 12, 18, 24, 36 months.
- Test for assay, impurities, dissolution, appearance, and microbial limits.
- Document everything. A typical stability dossier runs 450-600 pages.
- Validate your methods. If you can’t measure it accurately, you can’t prove stability.
Final Thoughts
Stability testing is one of the most tedious, expensive, and critical parts of bringing a drug to market. It’s not glamorous. But without it, patients could get pills that don’t work-or worse, pills that hurt them. The temperature and time conditions in ICH Q1A(R2) aren’t just bureaucratic boxes to check. They’re the scientific foundation of drug safety. As new therapies emerge, those standards will evolve. But the core principle won’t change: if you don’t know how your medicine behaves over time, you have no right to sell it.What are the standard temperature and humidity conditions for long-term stability testing?
The ICH Q1A(R2) guidelines define two options: 25°C ± 2°C with 60% RH ± 5% RH, or 30°C ± 2°C with 65% RH ± 5% RH. The choice depends on the climatic zone of the target market. For example, products sold in tropical regions (Zone IVa) must be tested under the 30°C/65% RH condition.
How long does accelerated stability testing last?
Accelerated stability testing runs for 6 months at 40°C ± 2°C and 75% RH ± 5% RH. This is not meant to reflect real-world storage but to predict long-term degradation under stress conditions. It helps identify potential stability issues early in development.
Is 6 months of long-term data enough for regulatory submission?
It depends on the region. The U.S. FDA requires 12 months of long-term data at submission. The European Medicines Agency (EMA) allows either 6 or 12 months, depending on the submission option selected. Companies seeking global approval often need to run both to avoid delays.
What is intermediate stability testing and when is it required?
Intermediate testing is conducted at 30°C ± 2°C and 65% RH ± 5% RH for 6 months. It’s only required when long-term testing is done at 25°C and accelerated testing shows a significant change. It helps bridge the gap between accelerated and long-term results, especially for products sensitive to humidity.
Do refrigerated drugs follow the same stability testing rules?
No. Refrigerated products are tested at 5°C ± 3°C for long-term storage and 25°C ± 2°C with 60% RH ± 5% RH for accelerated testing. This reflects the real risk for these products: accidental warming, not heat exposure. Freezing and thawing cycles are also tested separately for biologics and vaccines.
What happens if a stability chamber has a temperature excursion?
A temperature excursion outside ±2°C can invalidate an entire stability study. Most companies require chambers to be monitored continuously with alarms and backup systems. If an excursion occurs, the study may need to be restarted, which can delay product launch by months and cost hundreds of thousands of dollars.



3 Comments
So let me get this straight - we spend $200k+ and 3 years testing pills in a box that magically stays at 25°C and 60% humidity forever? In the real world, my cousin’s insulin sat in a hot car for 3 hours and somehow didn’t turn into soup. Guess what? It still worked. Maybe the rules are just corporate theater.
Also, who wrote this? It’s got more acronyms than a Pentagon memo. ICH Q1A(R2)? Sounds like a rejected Star Wars villain.
I work in a small pharma lab and I just want to say - this post is actually really well done. The part about humidity cycling? 100% true. We had a batch of tablets crack open last year because the warehouse doors were opened every 20 minutes during monsoon season. The lab report said ‘no significant change’ - but visually, they looked like they’d been through a tornado.
And yeah, the 5% assay drop rule? Totally arbitrary. One lab’s ‘failure’ is another’s ‘still perfectly safe’. We’ve had regulators argue over 0.3% impurity spikes like it’s a crime scene. The science is solid, but the interpretation? Pure chaos.
Also, shoutout to the guy who mentioned PAT - that tech is a game changer. We’re testing real-time moisture content now with NIR probes. It’s like having a weather station inside the pill. Wish we had this 10 years ago.
THEY’RE LYING TO YOU. ALL OF IT. The 40°C test? That’s not for safety - it’s for profit. Big Pharma doesn’t want you to know that most drugs are stable at room temp for 5+ years. They push ‘6-month accelerated’ so you buy new bottles every 12 months. Why? Because they make more money.
And don’t get me started on the ‘significant change’ definition. That’s a loophole. They can say ‘5% drop’ and still sell it - but if you look at the actual clinical data, the drug still works fine. They’re manipulating the rules to force replacements.
Remember the Vioxx scandal? Same playbook. They tested it ‘correctly’… then ignored the real-world data. Now they’re doing it again with biologics. Wake up.
And why is no one talking about the fact that these chambers are calibrated by the same companies that make the drugs? Conflict of interest much?
I’ve seen the internal memos. They know. They just don’t care.