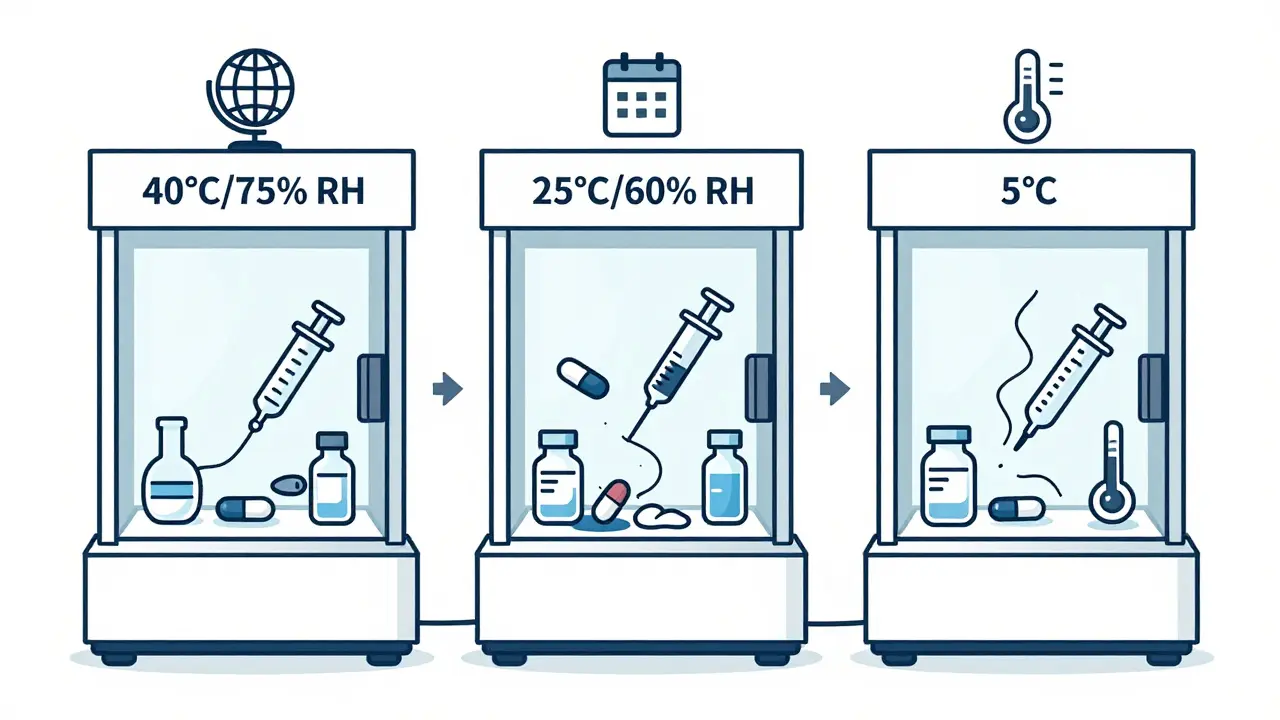
Stability testing ensures pharmaceutical products remain safe and effective over time. Learn the exact temperature and time conditions required by ICH Q1A(R2), including accelerated, long-term, and refrigerated protocols used globally.

Stability testing ensures pharmaceutical products remain safe and effective over time. Learn the exact temperature and time conditions required by ICH Q1A(R2), including accelerated, long-term, and refrigerated protocols used globally.