
Most people think the FDA can just pull a dangerous drug off the shelves the moment it’s found to be harmful. That’s not how it works. The truth is more complicated - and more concerning. The FDA doesn’t have the legal power to force a drug company to recall a medicine. It can only ask. And if the company says no? The FDA has to go to court.
How the FDA Actually Removes Unsafe Drugs
The FDA’s authority over drugs comes from the Federal Food, Drug, and Cosmetic Act (FD&C Act) of 1938. That law never gave the agency the right to order a recall. Instead, it lets the FDA request one. Manufacturers are expected to comply - and almost always do. But legally, it’s still a request, not a command.
When a drug is found to be unsafe, the FDA doesn’t swoop in and shut it down. It contacts the company, shares the evidence - maybe contaminated batches, unexpected side effects, or faulty labeling - and asks them to recall it. The company then decides whether to act. In 99.7% of cases, they do. But that 0.3% is where the system breaks down.
Take the 2018 valsartan recall. A cancer-causing impurity, NDMA, was found in a common blood pressure medication. The FDA issued a public alert on June 8, 2018. But the Chinese supplier that made the active ingredient didn’t respond for 17 days. The FDA couldn’t force them to act. It had to wait, issue more alerts, and pressure the U.S. manufacturers to pull their versions. It took weeks before the full market withdrawal was complete.
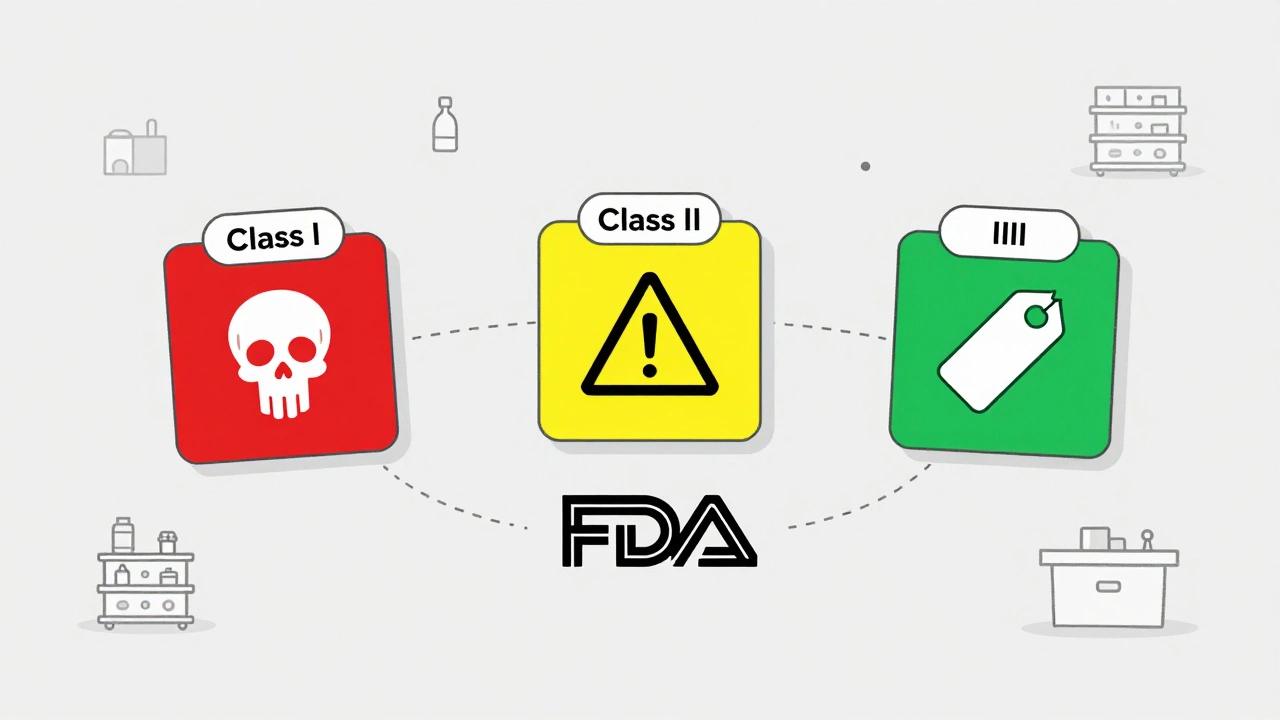
Class I, II, III: How Recalls Are Ranked by Risk
Not all recalls are the same. The FDA classifies them into three levels based on how dangerous the drug is.
- Class I recalls are the most serious. They involve products that could cause serious harm or death. In 2022, only 2.1% of all drug recalls fell into this category. These require immediate action - often within 24 hours of FDA notification.
- Class II recalls are more common. They involve products that might cause temporary or reversible health problems, or where the risk of serious harm is low. These made up nearly 70% of all recalls in 2022. Think wrong dosage labels or missing instructions.
- Class III recalls are the least urgent. The product won’t hurt you, but it violates FDA rules - maybe the bottle says “100 pills” but actually has 98. These are mostly about packaging or labeling errors.
The classification determines how far the recall goes. A Class I recall might need hospitals to notify every patient who got the drug. A Class III might just require the distributor to pull the batch from warehouse shelves.
Why the FDA Can’t Force Drug Recalls - But Can Force Device Recalls
Here’s the twist: the FDA can force recalls - but only for medical devices, not drugs.
Under 21 CFR 810, the FDA has direct legal authority to order a device recall if there’s a “reasonable probability” it could cause serious injury or death. That power came from the Medical Device Amendments of 1976, which gave the agency stronger tools for devices because they’re often implanted or used in critical care.
Drugs? Still stuck in the 1938 rulebook. That gap has drawn sharp criticism. Dr. Sidney Wolfe from Public Citizen testified before Congress in 2019 that the FDA’s inability to mandate drug recalls creates dangerous delays. He pointed to the valsartan case and others where companies dragged their feet.
But the FDA defends the system. In a 2021 interview, Deputy Commissioner Janet Woodcock said 99.7% of drug recalls happen voluntarily within 10 days of FDA notification. That sounds impressive - until you realize it’s only true when companies cooperate. What happens when they don’t?
What Happens When a Company Refuses to Recall
If a manufacturer refuses to recall a dangerous drug, the FDA doesn’t just walk away. It has to go to court.
Under Section 304 of the FD&C Act, the FDA can ask a federal judge to issue an injunction - a legal order that stops the company from making, shipping, or selling the drug. It’s a slow, expensive process. It can take months. And during that time, the drug stays on shelves.
Between 2012 and 2022, only 3 out of 15,241 drug recalls required FDA legal action. That’s a tiny number. But it’s not zero. And when it happens, people get hurt.
PhRMA, the drug industry’s main lobbying group, says the voluntary system works fine. They point to that 99.98% success rate. But critics say that’s not the point. The system relies on trust - and when trust fails, the consequences can be deadly.
How Hospitals and Pharmacies Handle Recalls
Even when the FDA and manufacturers act fast, the real challenge is on the ground - in hospitals, pharmacies, and doctor’s offices.
A 2022 survey by the American Society of Health-System Pharmacists (ASHP) found that 68% of hospital pharmacy directors struggled to identify which patients got recalled drugs. Why? Inconsistent lot numbering. One manufacturer uses a 10-digit code. Another uses letters and numbers. There’s no universal standard.
And 42% of pharmacies reported communication delays during Class I recalls. Patients weren’t notified for an average of 3.7 days after the recall was announced. In some cases, people kept taking the drug because their doctor never told them it was pulled.
ASHP recommends a 12-point system for hospitals: assign roles, track recalls daily, train staff regularly, and keep a central log of all recalled products. But not every hospital has the resources to do it right.
The 7 Million Recall Industry
Because the system is so fragmented, a whole industry has grown up around tracking recalls. Companies like Recall Masters and Recall Index sell software to hospitals and pharmacies that automatically alerts them when a drug is pulled.
In 2023, this market was worth $287 million. Seventy-three percent of U.S. hospitals use one of these services. Without them, many would be flying blind.
But here’s the irony: the FDA doesn’t require these tools. They’re optional. That means smaller clinics, rural pharmacies, and independent prescribers often don’t have access to timely alerts. They rely on emails, website checks, or word of mouth - none of which are reliable.
What’s Changing? The Push for Mandatory Recall Power
There’s been a push to fix this. The FD&C Modernization Act of 2022 included a provision - Section 604 - that would have given the FDA the power to order mandatory drug recalls. It was removed during committee markup. Why? Lobbying.
PhRMA spent $8.2 million in the second quarter of 2023 alone to fight any change that would give the FDA more power. Their argument? The system works. But experts like Dr. Peter Lurie of the Center for Science in the Public Interest say that’s a dangerous myth.
“The failure to grant the FDA explicit mandatory recall authority remains a critical vulnerability,” he wrote in a 2023 Health Affairs blog. “Especially for biologics, where contamination risks are rising.”
Another bill, the PREVENT Pandemics Act (S.2871), includes a section that would give the FDA mandatory recall power for drugs and biological products. As of October 2023, it’s still in committee. Industry opposition remains strong.
What You Can Do If You’re Taking a Recalled Drug
If you’re on medication, here’s how to protect yourself:
- Check the FDA’s Recalls page monthly - even if you feel fine.
- Know your drug’s lot number. It’s on the bottle or packaging. Write it down.
- Don’t wait for your doctor to call. If you hear a recall, contact your pharmacy immediately.
- If you’re on a Class I recalled drug, stop taking it and call your doctor right away - don’t wait.
- Use the MedWatch program to report side effects. The FDA gets over a million reports a year. Your report could be the one that triggers a recall.
The system isn’t broken - it’s just outdated. The FDA can’t force a recall. But it can still save lives - by acting fast, speaking clearly, and pushing for change. Until then, the burden falls on patients, pharmacists, and doctors to stay alert. Because no one else will.



8 Comments
so the fda can’t even force a recall?? lol. they’re like a mom asking her kid to clean their room and then just sighing when they say ‘no’. meanwhile people are dropping dead from tainted blood pressure meds and the agency’s like ‘well we asked nicely’ 🤡
The structural asymmetry in regulatory authority between pharmaceuticals and medical devices is a glaring legislative artifact. The FD&C Act of 1938 was never designed for the pharmacovigilance demands of 21st-century biologics. The absence of mandatory recall power constitutes a systemic risk vector - particularly for Class I agents with high penetrance in outpatient populations. The 0.3% non-compliance rate is statistically insignificant, but clinically catastrophic.
Yeah I get what you’re saying about the FDA not being able to force recalls… but honestly, most companies do the right thing. I mean, 99.7% is pretty good, right? And if they don’t, the FDA can go to court - it’s just slower. Maybe we just need better communication between pharmacies and patients instead of giving the FDA more power. People just need to check their lot numbers more often.
For hospitals and pharmacies, the real bottleneck isn’t the FDA’s authority - it’s the lack of standardized lot numbering and fragmented alert systems. Implementing a unified, FDA-mandated barcode protocol across all manufacturers would solve 80% of the problem. Pair that with mandatory staff training and automated MedWatch integration, and you reduce response times dramatically. This isn’t about power - it’s about interoperability.
they’ve been doing this on purpose. the fda doesn’t have power because the pharmaceutical lobby owns congress. you think it’s an accident that the 2022 bill got stripped? nah. it’s all connected. the same people who make your blood pressure meds also fund your senator’s yacht. and now you’re supposed to trust them? lol. next they’ll say ‘just pray harder’ when your kid gets cancer from a tainted batch. i’ve seen the documents. they knew. they always knew.
It is, regrettably, a matter of considerable concern that the regulatory framework governing pharmaceutical recalls remains rooted in legislation predating the advent of modern pharmacology. The absence of statutory authority to mandate recalls constitutes, in my estimation, a manifest dereliction of duty by the legislative branch. One might reasonably posit that the current system is not merely antiquated, but actively perilous.
so the fda can't force a recall but can force a device recall?? sounds like they think your heart stent is more important than your life lol. i took valsartan for 3 years and no one told me. my pharmacist didn't even know. just me googling 'why do i feel like i'm dying' and finding out i was on a cancer drug 🤷♀️
lol i just checked my blood pressure med and the lot number is 7B-24KX9… i have no idea if that’s recalled 😅 maybe i should call my pharmacy? or just keep taking it and hope for the best? 🤔 also anyone know if there’s an app for this? i don’t wanna google every time i refill