When a pharmacist fills a prescription for a generic drug, they don't just guess whether it's safe to swap it for the brand name. They check the FDA Orange Book. This isn't just a reference guide-it's the official source that tells pharmacies, doctors, and insurers which generic drugs can be substituted without risking patient safety. If you're a pharmacist, a patient, or even a healthcare student, knowing how to use this tool correctly can prevent errors, save money, and ensure treatment works as intended.
What Is the FDA Orange Book?
The FDA Orange Book, officially called Approved Drug Products with Therapeutic Equivalence Evaluations, is published by the U.S. Food and Drug Administration. It started in 1980 but became legally required after the 1984 Hatch-Waxman Act. That law created a faster path for generic drugs to enter the market, but only if they proved they worked the same as the brand-name version. The Orange Book is how the FDA keeps track of which generics pass that test.
It lists over 16,000 approved drug products, including both prescription and some over-the-counter medications. But here's the key: only prescription drugs get therapeutic equivalence ratings. OTC drugs are not evaluated in the Orange Book. That means if you're looking up something like ibuprofen or loratadine, you won't find a TE code for them-even though they’re sold as generics.
The electronic version, called the Electronic Orange Book, updates daily. The last full revision was in September 2023. It’s free, public, and accessible through the FDA website. No login. No fee. Just search, find, and verify.
Understanding Therapeutic Equivalence (TE) Codes
The heart of the Orange Book is its two-letter TE codes. These aren’t random labels-they’re scientific judgments. Each code tells you whether a generic drug can be safely swapped for the brand.
- AB: This is the gold standard. It means the generic has been proven bioequivalent to the reference drug. Same active ingredient, same dose, same form, and it works the same way in the body. Most generics you see in pharmacies are AB-rated.
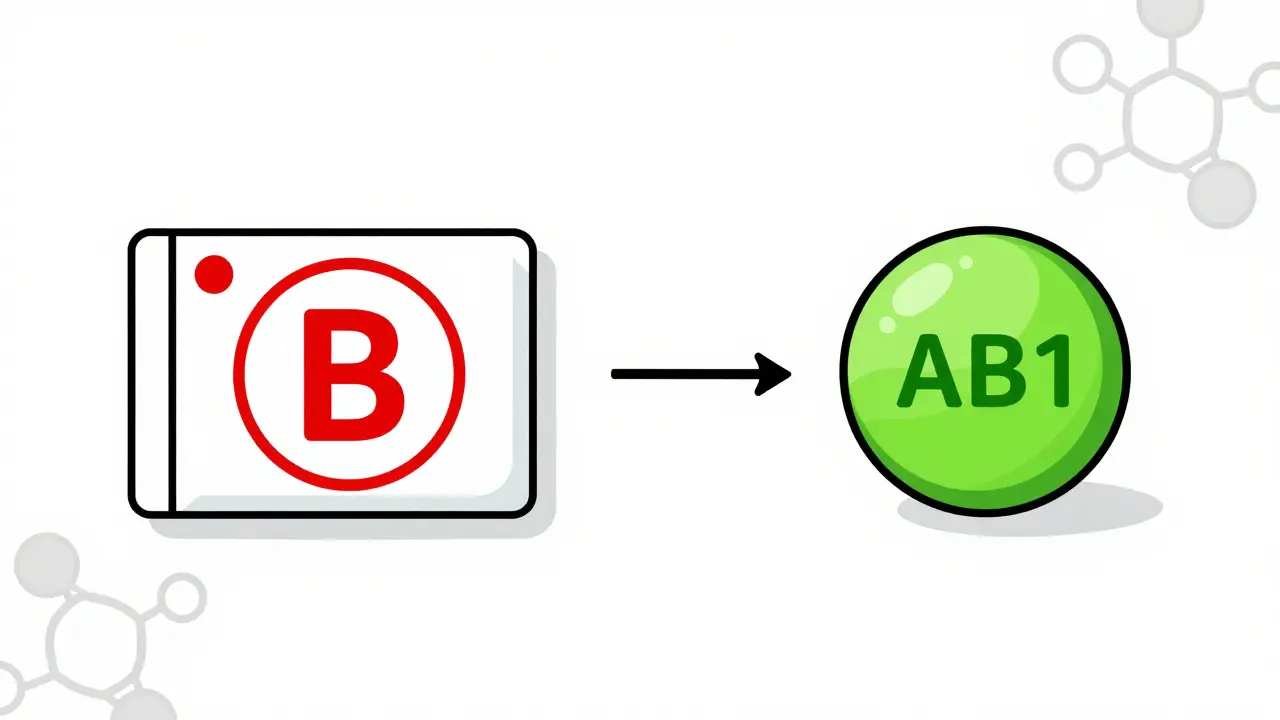
- AB1, AB2, AB3: These mean the generic matches one of multiple reference drugs for the same active ingredient. For example, if there are three different brand versions of a drug, each might have its own AB code. You must match the AB number to the correct reference listed drug (RLD).
- B: This is a red flag. It means the generic has not been proven equivalent. It might have different absorption, inconsistent release, or untested formulation issues. Pharmacies cannot substitute these without a doctor’s order.
- BX: This is a newer code for drugs with potential bioequivalence concerns. It doesn’t mean the drug is unsafe, but the FDA doesn’t recommend automatic substitution. These are often complex products like inhalers, topical creams, or injectables.
According to the FDA, a drug gets an AB rating only if it meets five strict criteria: approved as safe and effective, pharmaceutically equivalent, bioequivalent, properly labeled, and made under good manufacturing practices. No exceptions.
How to Search the Electronic Orange Book
Here’s how to verify a generic drug step by step. This process works whether you’re checking for a patient, a formulary decision, or just learning.
- Start with the brand name. Go to the Electronic Orange Book website. In the search bar, type the brand name-for example, “Synthroid.” Click Search.
- Find the Reference Listed Drug (RLD). The results will show multiple entries. Look for the one marked “Yes” under the RLD column. That’s the original brand drug the generic was designed to match. Note its active ingredient, dosage form, and strength.
- Search by active ingredient. Now go to the Ingredient Search. Type in the active ingredient-like “levothyroxine.” Filter by the same dosage form (e.g., tablet, oral) and route (oral).
- Check the TE code column. All generic versions will appear here. Look for the AB rating next to each. If it says AB1, make sure it matches the RLD you found earlier. If it says B or BX, don’t assume it’s interchangeable.
- Verify the manufacturer and applicant number. Each product has an application number (e.g., ANDA 205678). This helps you track which company made it and whether there are patent issues that might affect availability.
Pro tip: If you don’t see a TE code at all, the product might be discontinued. Check the Discontinued Drug Product List-it’s a separate tab on the site. Also, avoid using third-party apps like Drugs.com or IBM Micromedex for critical decisions. The FDA says they can be 24 to 72 hours behind the official database.
Why TE Codes Matter in Real-World Practice
It’s not just about science. It’s about law and practice. In 49 states, pharmacists can substitute a generic for a brand if the Orange Book says AB. But in one state-California-the rules are stricter. Some drugs, even with AB ratings, require a doctor’s note for substitution if they’re in the narrow therapeutic index (NTI) category.
Drugs like warfarin, levothyroxine, and phenytoin fall into this category. Small differences in absorption can lead to serious side effects. The Orange Book doesn’t flag NTI drugs. That’s on the pharmacist to know. A 2022 survey by the American Pharmacists Association found that 42% of pharmacists struggled with interpreting TE codes for complex products like inhalers and topical gels.
Another common mistake: confusing patent expiration with market exclusivity. A drug might lose its patent, but still have 180 days of exclusivity granted to the first generic applicant. During that time, no other generics can enter-even if they’re AB-rated. The Orange Book lists both, but you have to read carefully.
Common Errors and How to Avoid Them
Even experienced professionals make mistakes. Here are the top three:
- Mixing up AB1 and AB2. If a drug has two different reference versions (say, one from Brand A and one from Brand B), the generics for each will have different codes. Substituting an AB1 for an AB2 could mean giving a patient a different formulation than prescribed.
- Assuming all generics are equal. Just because two generics are AB-rated doesn’t mean they’re identical. One might use a different filler or coating. That can matter for patients with allergies or absorption issues.
- Ignoring discontinued products. If a brand drug was pulled from the market, its generics might still be listed-but without an RLD. That means they can’t be legally substituted anymore.
The FDA recommends doing at least five verifications before feeling confident. Start with simple drugs like metformin or lisinopril. Then move to trickier ones like levothyroxine or warfarin. Use their free 12-page Quick Reference Guide (updated March 2023) and watch their Drug Info Rounds webinars. Most pharmacists say they feel 70% more confident after watching just one tutorial.
What’s Next for the Orange Book?
The FDA is moving toward more automation. By 2024, they plan to integrate the Orange Book with the Purple Book (which tracks biologics). They’re also switching to structured product labeling (SPL), which lets software directly pull data instead of manual searches.
By 2027, Evaluate Pharma predicts 70% of generic verifications will happen through API integrations-like electronic health records or pharmacy management systems pulling live Orange Book data. But for now, the manual search is still the gold standard. If you’re making decisions about substitutions, don’t rely on automation. Go to the source.
Final Thoughts
The FDA Orange Book isn’t just a list of drugs. It’s a legal and clinical safeguard. It keeps patients safe, ensures fair competition, and keeps costs down. But it only works if you use it correctly.
Don’t skip the steps. Don’t assume. Don’t rely on third-party tools. Always check the official site. If you’re unsure, email [email protected]. The FDA responds to 95% of questions in under 48 hours.
At its core, the Orange Book answers one question: Can this generic be swapped without risk? The answer is written in those two letters: AB. Learn how to read them. Your patients will thank you.
What does an AB rating mean in the FDA Orange Book?
An AB rating means the generic drug has been determined by the FDA to be therapeutically equivalent to the brand-name reference drug. This means it contains the same active ingredient, in the same strength and dosage form, and has been proven bioequivalent through testing. Pharmacists can substitute AB-rated generics without needing a doctor’s approval in most states.
Are all generic drugs listed in the Orange Book?
No. Only prescription drugs with FDA-approved Abbreviated New Drug Applications (ANDAs) are listed. Over-the-counter (OTC) drugs are not evaluated for therapeutic equivalence and do not have TE codes. Also, discontinued products appear in a separate list and are not included in the main therapeutic equivalence data.
Can I trust third-party websites like Drugs.com for Orange Book data?
No-not for critical decisions. While third-party sites like Drugs.com and IBM Micromedex use Orange Book data, they can be 24 to 72 hours behind the official FDA database. For legal, clinical, or insurance purposes, always verify directly on the FDA’s Electronic Orange Book website. The FDA explicitly warns against relying on delayed sources.
Why do some generics have AB1, AB2, or AB3 ratings?
These numbers indicate which specific reference listed drug (RLD) the generic matches. Some drugs have more than one approved brand version (RLDs). Each RLD may have different inactive ingredients or formulations. The AB1, AB2, AB3 codes ensure that generics are only substituted for the exact RLD they were tested against. Mixing AB1 with AB2 could mean switching to a different formulation.
Does the Orange Book tell me if a drug is safe for substitution in all patients?
No. The Orange Book only confirms therapeutic equivalence based on average patient response. It does not account for individual factors like allergies, absorption issues, or narrow therapeutic index (NTI) concerns. For drugs like levothyroxine or warfarin, even small differences can matter. State laws and clinical judgment must be used alongside the Orange Book to ensure safe substitution.